Рефераты по авиации и космонавтике
Рефераты по административному праву
Рефераты по безопасности жизнедеятельности
Рефераты по арбитражному процессу
Рефераты по архитектуре
Рефераты по астрономии
Рефераты по банковскому делу
Рефераты по сексологии
Рефераты по информатике программированию
Рефераты по биологии
Рефераты по экономике
Рефераты по москвоведению
Рефераты по экологии
Краткое содержание произведений
Рефераты по физкультуре и спорту
Топики по английскому языку
Рефераты по математике
Рефераты по музыке
Остальные рефераты
Рефераты по биржевому делу
Рефераты по ботанике и сельскому хозяйству
Рефераты по бухгалтерскому учету и аудиту
Рефераты по валютным отношениям
Рефераты по ветеринарии
Рефераты для военной кафедры
Рефераты по географии
Рефераты по геодезии
Рефераты по геологии
Рефераты по геополитике
Рефераты по государству и праву
Рефераты по гражданскому праву и процессу
Рефераты по кредитованию
Рефераты по естествознанию
Рефераты по истории техники
Рефераты по журналистике
Рефераты по зоологии
Рефераты по инвестициям
Рефераты по информатике
Исторические личности
Рефераты по кибернетике
Рефераты по коммуникации и связи
Рефераты по косметологии
Рефераты по криминалистике
Рефераты по криминологии
Рефераты по науке и технике
Рефераты по кулинарии
Рефераты по культурологии
Курсовая работа: Ecological problems. Environmental protection
Курсовая работа: Ecological problems. Environmental protection
Министерство образования и науки Украины
Днепропетровский областной медицинский
Лицей – интернат «Днiпро»
Курсовая работа
«Ecological problems. Environmental protection»
Выполнила:
Ученица 11 – Г класса
Калашникова Анастасия
Научные руководители:
Кривонос И.А.
Легкий П.В.
Introduction
My term-paper is devoted to the theme of the global ecological problems and the environmental protection. I would like to tell you about some problems for example “Greenhouse effect”. The aim of my project is to show and explain how ecological problems influence on our life and about there consequences.
The sources of my work are:
· Scientific books and newspapers
· Numerous internet data
My project consists of the following parts: Introduction, Literature overview, conclusion and literature.
· Literature overview consists of 11 themes.
· Conclusion.
· Literature.
I suppose that the topic I chose is very actual nowadays and I hope that it will contribute to our knowledge and will also have a practical implementation in the class.
Ecological situation nowadays
Ecology is a very popular word today. But what does it mean? Ecology is a since which studies the relationship between all forms of life on our planet and the environment. This word came from Greek “oikos” which means home. The idea of home includes our whole planet, its population, Nature, animals, birds, fish, insects and all other living beings and even the atmosphere around our planet.
Since ancient times Nature has served Man giving everything he needs: air to breathe, food to eat, water to drink, wood for building and fuel for heating his home. For thousands of years people lived in harmony with the environment and it seemed to them that the resources of nature had no end or limit. With the industrial revolution our negative influence on Nature began to increase. Large cities with thousands of steaming, polluting plants and factories can be found nowadays all over the world. The by-products of their activity pollute the air we breathe the water we drink the fields where our crops are grown. That’s why those who live in cities prefer spending their days off and their holidays far from the noise of the city, to be closer to nature. Perhaps they like to breathe fresh air or to swim in clear water because the ecology is not so poor as in the cities.
So, pollution is one of the most burning problems of nowadays. Now millions of chimneys, cars, buses, trucks all over the world exhaust fumes and harmful substances into the atmosphere. These poisoned substances pollute everything: air, land, water, birds and animals. So, it is usually hard to breathe in the large cities where there are lots plants.
Every year the atmosphere is polluted by about 1000 tons of industrial dust and other harmful substances. Big cities suffer from smog. Cars with their engine have become the main source of pollution in industrial countries. Vast forests are being cut down for the need of industries in Europe and USA. The loss of the forests upsets the oxygen balance of the new wastelands. As the result some species of animals, birds, fish and plants have disappeared and keep disappearing.
Water pollution is very serious, too. Ugly rivers of dirty water polluted with factory waste, poisoned fish are all-round us. And polluted air and poisoned water lead to the end of the civilization. So, nowadays a lot of dead lands and lifeless areas have appeared, because our actions and dealings can turn the land to a desert.
Greenhouse effect
The greenhouse effect is the process in which the emission of infrared radiation by the atmosphere warms a planet's surface. The name comes from an analogy with the warming of air inside a greenhouse compared to the air outside the greenhouse. The Earth's average surface temperature is about 33°C warmer than it would be without the greenhouse effect. The greenhouse effect was discovered by Joseph Fourier in 1829 and first investigated quantitatively by Svante Arrhenius in 1896. In addition to the Earth, Mars and especially Venus have greenhouse effects.


Basic mechanism
ecological environmental protection greenhouse
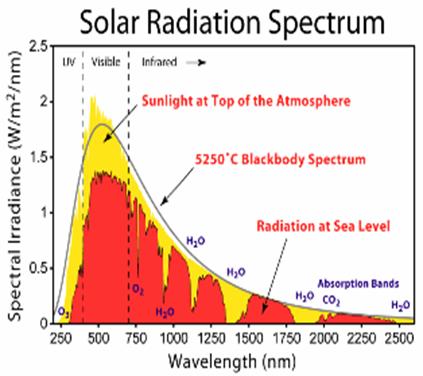
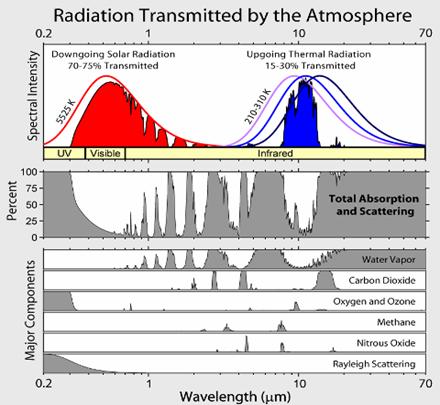
The Earth receives energy from the Sun in the form of radiation. The Earth reflects about 30% of the incoming solar radiation. The remaining 70% is absorbed, warming the land, atmosphere and oceans. For the Earth's temperature to be in steady state so that the Earth does not rapidly heat or cool, this absorbed solar radiation must be very nearly balanced by energy radiated back to space in the infrared wavelengths. Since the intensity of infrared radiation increases with increasing temperature, one can think of the Earth's temperature as being determined by the infrared flux needed to balance the absorbed solar flux. The visible solar radiation mostly heats the surface, not the atmosphere, whereas most of the infrared radiation escaping to space is emitted from the upper atmosphere, not the surface. The infrared photons emitted by the surface are mostly absorbed in the atmosphere by greenhouse gases and clouds and do not escape directly to space.
The reason this warms the surface is most easily understood by starting with a simplified model of a purely radiative greenhouse effect that ignores energy transfer in the atmosphere by convection (sensible heat transport) and by the evaporation and condensation of water vapor (latent heat transport). In this purely radiative case, one can think of the atmosphere as emitting infrared radiation both upwards and downwards. The upward infrared flux emitted by the surface must balance not only the absorbed solar flux but also this downward infrared flux emitted by the atmosphere. The surface temperature will rise until it generates thermal radiation equivalent to the sum of the incoming solar and infrared radiation.
A more realistic picture taking into account the convective and latent heat fluxes is somewhat more complex. But the following simple model captures the essence. The starting point is to note that the opacity of the atmosphere to infrared radiation determines the height in the atmosphere from which most of the photons are emitted into space. If the atmosphere is more opaque, the typical photon escaping to space will be emitted from higher in the atmosphere, because one then has to go to higher altitudes to see out to space in the infrared. Since the emission of infrared radiation is a function of temperature, it is the temperature of the atmosphere at this emission level that is effectively determined by the requirement that the emitted flux balance the absorbed solar flux.
But the temperature of the atmosphere generally decreases with height above the surface, at a rate of roughly 6.5 °C per kilometer on average, until one reaches the stratosphere 10-15 km above the surface. (Most infrared photons escaping to space are emitted by the troposphere, the region bounded by the surface and the stratosphere, so we can ignore the stratosphere in this simple picture.) A very simple model, but one that proves to be remarkably useful, involves the assumption that this temperature profile is simply fixed, by the non-radiative energy fluxes. Given the temperature at the emission level of the infrared flux escaping to space, one then computes the surface temperature by increasing temperature at the rate of 6.5 °C per kilometer, the environmental lapse rate, until one reaches the surface. The more opaque the atmosphere, and the higher the emission level of the escaping infrared radiation, the warmer the surface, since one then needs to follow this lapse rate over a larger distance in the vertical. While less intuitive than the purely radiative greenhouse effect, this less familiar radiative-convective picture is the starting point for most discussions of the greenhouse effect in the climate modeling literature.
Greenhouse gases
Quantum mechanics provides the basis for computing the interactions between molecules and radiation. Most of this interaction occurs when the frequency of the radiation closely matches that of the spectral lines of the molecule, determined by the quantization of the modes of vibration and rotation of the molecule. (The electronic excitations are generally not relevant for infrared radiation, as they require energy larger than that in an infrared photon.)
The width of a spectral line is an important element in understanding its importance for the absorption of radiation. In the Earth’s atmosphere these spectral widths are primarily determined by “pressure broadening”, which is the distortion of the spectrum due to the collision with another molecule. Most of the infrared absorption in the atmosphere can be thought of as occurring while two molecules are colliding. The absorption due to a photon interacting with a lone molecule is relatively small. This three-body aspect of the problem, one photon and two molecules, makes direct quantum mechanical computation for molecules of interest more challenging. Careful laboratory spectroscopic measurements provide the basis for most of the radioactive transfer calculations used in studies of the atmosphere.
The molecules/atoms that constitute the bulk of the atmosphere: oxygen (O2), nitrogen (N2) and argon; do not interact with infrared radiation significantly. While the oxygen and nitrogen molecules can vibrate, because of their symmetry these vibrations do not create any transient charge separation. Without such a transient dipole moment, they can neither absorb nor emit infrared radiation. In the Earth’s atmosphere, the dominant infrared absorbing gases are water vapor, carbon dioxide, and ozone (O3). The same molecules are also the dominant infrared emitting molecules. CO2 and O3 have "floppy" vibration motions whose quantum states can be excited by collisions at energies encountered in the atmosphere. For example, carbon dioxide is a linear molecule, but it has an important vibrational mode in which the molecule bends with the carbon in the middle moving one way and the oxygens on the ends moving the other way, creating some charge separation, a dipole moment, thus carbon dioxide molecules can absorb IR radiation. Collisions will immediately transfer this energy to heating the surrounding gas. On the other hand, other CO2 molecules will be vibrationally excited by collisions. Roughly 5% of CO2 molecules are vibrationally excited at room temperature and it is this 5% that radiates. A substantial part of the greenhouse effect due to carbon dioxide exists because this vibration is easily excited by infrared radiation. CO2 has two other vibrational modes. The symmetric stretch does not radiate, and the asymmetric stretch is at too high a frequency to be effectively excited by atmospheric temperature collisions, although it does contribute to absorption of IR radiation. The vibrational modes of water are at too high energies to effectively radiate, but do absorb higher frequency IR radiation. Water vapor has a bent shape. It has a permanent dipole moment (the O atom end is electron rich, and the H atoms electron poor) which means that IR light can be emitted and absorbed during rotational transitions, and these transitions can also be produced by collisional energy transfer. Clouds are also very important infrared absorbers. Therefore, water has multiple effects on infrared radiation, through its vapor phase and through its condensed phases. Other absorbers of significance include methane, nitrous oxide and the chlorofluorocarbons.
Discussion of the relative importance of different infrared absorbers is confused by the overlap between the spectral lines due to different gases, widened by pressure broadening. As a result, the absorption due to one gas cannot be thought of as independent of the presence of other gases. One convenient approach is to remove the chosen constituent, leaving all other absorbers, and the temperatures, untouched, and monitoring the infrared radiation escaping to space. The reduction in infrared absorption is then a measure of the importance of that constituent. More precisely, define the greenhouse effect (GE) to be the difference between the infrared radiation that the surface would radiate to space if there were no atmosphere and the actual infrared radiation escaping to space. Then compute the percentage reduction in GE when a constituent is removed. The table below is computed by this method, using a particular 1-dimensional model of the atmosphere. More recent 3D computations lead to similar results.
|
Gas removed |
percent reduction in GE |
|
H2O CO2 O3 |
36% 12% 3% |
By this particular measure, water vapor can be thought of as providing 36% of the greenhouse effect, and carbon dioxide 12%, but the effect of removal of both of these constituents will be greater than 48%. An additional proviso is that these numbers are computed holding the cloud distribution fixed. But removing water vapor from the atmosphere while holding clouds fixed is not likely to be physically relevant. In addition, the effects of a given gas are typically nonlinear in the amount of that gas, since the absorption by the gas at one level in the atmosphere can remove photons that would otherwise interact with the gas at another altitude. The kinds of estimates presented in the table, while often encountered in the controversies surrounding global warming, must be treated with caution. Different estimates found in different sources typically result from different definitions and do not reflect uncertainties in the underlying radioactive transfer.
When Do You Send Greenhouse Gases into the Air
Whenever you...
Watch TVUse a Hair Dryer
Use the Air ConditionerRide in a Car
Turn on a LightPlay a Video Game
Listen to a StereoWash or Dry Clothes
Use a Dish WasherMicrowave a Meal
... you are helping to send greenhouse gas into the air.
To perform many of these functions, you need to use electricity. Electricity comes from power plants. Most power plants use coal and oil to make electricity. Burning coal and oil produces greenhouse gases.
Other things we do send greenhouse gases into the air
The trash that we send to landfills produces a greenhouse gas called methane. Methane is also produced by the animals we raise for dairy and meat products and when we take coal out of the ground. Whenever we drive or ride in a car, we are adding greenhouse gases to the atmosphere. And, when factories make the things that we buy and use everyday, they too are sending greenhouse gases into the air.
And now let’s talk about Climate and Weather
Weather is all around us. Weather may be one of the first things you notice after you wake up. Changes are, if it is cold and snowing, you'll wear a jacket when you go outside. If it's hot and sunny, you may wear shorts. Sounds pretty simple, right?
But what about climate? How is it different from weather? And what is weather, exactly?
Weather
Weather describes whatever is happening outdoors in a given place at a given time. Weather is what happens from minute to minute. The weather can change a lot within a very short time. For example, it may rain for an hour and then become sunny and clear. Weather is what we hear about on the television news every night. Weather includes daily changes in precipitation, barometric pressure, temperature, and wind conditions in a given location.
Climate
Climate describes the total of all weather occurring over a period of years in a given place. This includes average weather conditions, regular weather sequences (like winter, spring, summer, and fall), and special weather events (like tornadoes and floods). Climate tells us what it's usually like in the place where you live. San Diego is known as having a mild climate, New Orleans a humid climate, Buffalo a snowy climate, and Seattle a rainy climate.
Is the climate warming
Global surface temperatures have increased about 0.6°C (plus or minus 0.2°C) since the late-19th century, and about one half degree F (0.2 to 0.3°C) over the past 25 years (the period with the most credible data). The warming has not been globally uniform. Some areas (including parts of the southeastern U.S.) have cooled. The recent warmth has been greatest over N. America and Eurasia between 40 and 70°N. Warming, assisted by the record El Nino of 1997-1998, has continued right up to the present. Linear trends can vary greatly depending on the period over which they are computed. Temperature trends in the lower troposphere (between about 2,500 and 18,000 ft.) from 1979 to the present, the period for which Satellite Microwave Sounding Unit data exist, are small and may be unrepresentative of longer term trends and trends closer to the surface. Furthermore, there are small unresolved differences between radiosonde and satellite observations of tropospheric temperatures, though both data sources show slight warming trends. If one calculates trends beginning with the commencement of radiosonde data in the 1950s, there is a slight greater warming in the record due to increases in the 1970s. There are statistical and physical reasons (e.g., short record lengths, the transient differential effects of volcanic activity and El Nino, and boundary layer effects) for expecting differences between recent trends in surface and lower tropospheric temperatures, but the exact causes for the differences are still under investigation (see National Research Council report "Reconciling Observations of Global Temperature Change").
An enhanced greenhouse effect is expected to cause cooling in higher parts of the atmosphere because the increased "blanketing" effect in the lower atmosphere holds in more heat. Cooling of the lower stratosphere (about 30-35,000ft.) since 1979 is shown by both satellite Microwave Sounding Unit and radiosonde data, but is larger in the radiosonde data.
There has been a general, but not global, tendency toward reduced diurnal temperature range (the difference between high and low daily temperatures) over about 50% of the global land mass since the middle of the 20th century. Cloud cover has increased in many of the areas with reduced diurnal temperature range.
Relatively cool surface and tropospheric temperatures, and a relatively warmer lower stratosphere, were observed in 1992 and 1993, following the 1991 eruption of Mt. Pinatubo. The warming reappeared in 1994. A dramatic global warming, at least partly associated with the record El Nino, took place in 1998. This warming episode is reflected from the surface to the top of the troposphere. Indirect indicators of warming such as borehole temperatures, snow cover, and glacier recession data, are in substantial agreement with the more direct indicators of recent warmth.
Arctic sea ice has decreased since 1973, when satellite measurements began but Antarctic sea ice may have increased slightly.
Can we change the climate
It may seem hard to believe that people can actually change the Earth’s climate. But scientists think that the things people do that send greenhouse gases into the air are making our planet warmer.
Once, all climate changes occurred naturally. However, during the Industrial Revolution, we began altering our climate and environment through agricultural and industrial practices. The Industrial Revolution was a time when people began using machines to make life easier. It started more than 200 years ago and changed the way humans live. Before the Industrial Revolution, human activity released very few gases into the atmosphere, but now through population growth, fossil fuel burning, and deforestation, we are affecting the mixture of gases in the atmosphere.
Since the Industrial Revolution, the need for energy to run machines has steadily increased. Some energy, like the energy you need to do your homework, comes from the food you eat. But other energy, like the energy that makes cars run and much of the energy used to light and heat our homes, comes from fuels like coal and oil – fossil fuels. Burning these fuels releases greenhouse gases.
Environmental protection in Ukraine
In the 20th century, the rapid growth of science and technology resulted in an increasing negative effect on the biosphere of the Earth. Huge industrial enterprises pollute the air we breathe? The water we drink and the land, which gives us bread, vegetables, and fruit. Their discharge of dust and gas into the atmosphere returns to the Earth in the form of acid rains. It also destroys the ozone layer of the Earth and causes ‘’ greenhouse effect‘’. It effects forests, rivers, crops and people’s health. This leads to the reduction of the life-span of man. People die younger because of cancer, AIDS and other diseases which are directly connected with the polluted environment they live in. Many species of animals and birds face extinction due to the pollution of the biosphere.
The world’s oceans are in danger too. They are filled with poisonous industrial and nuclear waste, chemical fertilizers and pesticides. The Aral Sea in Russia is already dead, the Mediterranean and the North Sea are slowly dying.
The worst situation with air pollution is in big overpopulated cities. In Cairo and Mexico City, for example, breathing is equivalent to smoking 2 packs of cigarettes a day. The big industrial cities in Ukraine like Zaporizhiya, Donetsk, Kharkiv and some others have the same situation.
Another threat for the environment are nuclear power stations like Chernobyl. In April 1986 that nuclear power plant just north-west of Kyiv suffered the worst nuclear accident in history: dozens died immediately, tens of thousands were evacuated, while the long-term effects to human life are difficult to calculate. A large part of Ukraine, Russia and Byelorussia was polluted by radioactive substances. Great damage was done to their economy, nature and people’s health. The problem of Chernobyl has not been solved yet because of the economic difficulties that Ukraine is having now. The power plant was closed on December 15, 2000.
Nowadays people of Ukraine, like most people in developed countries, realize that without solving environmental problems, the life of the future generations will be in real danger. Many people join the Great Party of Ukraine to unite their efforts to save the planet where we live, to make our world healthier and more beautiful.
Greenpeace
Greenpeace is an international environmental organization founded in Vancouver, British Columbia, Canada in 1971. It is best known for its campaigns against whaling. In later years, the focus of the organization turned to other environmental issues, including bottom trawling, global warming, ancient forest destruction, nuclear power, and genetic engineering. Greenpeace has national and regional offices in 42 countries worldwide, all of which are affiliated to the Amsterdam-based Greenpeace International. The global organization receives its income through the individual contributions of an estimated 2.8 million financial supporters, as well as from grants from charitable foundations, but does not accept funding from governments or corporations.
Mission statement
Greenpeace's official mission statement describes the organization and its aims thus:
Greenpeace is an independent, campaigning organization which uses peaceful direct action and creative communication to expose global environmental problems, and to force solutions for a green and peaceful future. Greenpeace's goal is to ensure the ability of the earth to nurture life in all its diversity.
Structure
Greenpeace is a global environmental organization, consisting of Greenpeace International (Stichting Greenpeace Council) in Amsterdam, and 27 national and regional offices around the world, providing a presence in 41 countries. These national and regional offices are largely autonomous in carrying out jointly agreed global campaign strategies within the local context they operate in, and in seeking the necessary financial support from donors to fund this work. National and regional offices support a network of volunteer-run local groups. Local groups participate in campaigns in their area, and mobilise for larger protests and activities elsewhere. Millions of supporters who are not organized into local groups support Greenpeace by making financial donations and participating in campaigns as citizens and consumers.
National and regional offices
Greenpeace is present in the following countries and regions, as of March 2007: Argentina, Australia-Pacific region (Australia, Fiji, Papua New-Guinea, Solomon Islands), Belgium, Brazil, Canada, Chile, China, Czech Republic, France, Germany, Greenpeace Nordic (Denmark, Finland, Norway, Sweden), Greece, Greenpeace Central and Eastern Europe (Austria, Hungary, Slovak Republic, Poland, Romania, Bulgaria, Slovenia, Serbia, Montenegro and Bosnia), India, Italy, Japan, Luxembourg, Greenpeace Mediterranean (Israel, Cyprus, Lebanon, Malta, Tunisia, Turkey), Mexico, the Netherlands, Greenpeace Aotearoa New Zealand (New Zealand), Russia, South-East Asia (Philippines, Indonesia, Thailand), Spain, Switzerland, United Kingdom, and the United States.
Friend of Earth (FoE)
Friends of the Earth is the U.S. voice of an influential, international network of grassroots groups in 70 countries. Founded in San Francisco in 1969 by David Brower, Friends of the Earth has for decades been at the forefront of high-profile efforts to create a more healthy, just world. There members were the founders of what is now the world's largest federation of democratically elected environmental groups, Friends of the Earth International.
In March of 2005, Friends of the Earth finalized a merger with Bluewater Network. Bluewater is a dynamic organization with creative campaigns to combat global warming, air and water pollution and damage to public lands by thrill vehicles such as snowmobiles and jetskis. The merger has added to our capacity and enabled us to broaden the scope of our work in a number of areas.
Among there present efforts are successes that draw headlines nationwide and international and local efforts that make a difference in your backyard and those of people a world away.
FoE conducted lab tests that confirmed our suspicion that genetically engineered corn not approved for human consumption was in products on supermarket shelves across the nation.
They also exposed the fact that Enron received $2.5 billion in taxpayer loans funneled through international financial institutions.
In Indiana, they are working with local groups to fight the destructive new-terrain I-69 project. This 140-mile, $1.8 billion highway would demolish thousands of acres of farms and forests and bisect an Amish community.
Over the years, there efforts and those of our supporters mean FoE have been able to: stop over 150 bad dams and water projects worldwide; ban international whaling; oust infamous James Watt; press for landmark regulations of strip mining and oil tankers; reform the World Bank; and eliminate billions in taxpayer subsidies to corporate polluters.
Literature
Internet data:
· www.greenpeace.com
· www.world-ecology.com
· http://en.wikipedia.org/wiki/Greenhouse_effect#_note-2
· http://epa.gov/climatechange/kids/climateweather.html
· http://epa.gov/climatechange/kids/change.html
· www.google.com.ua
Multimedia Editions
· Britannica Encyclopedia (Multimedia Edition)
· British Multimedia Encyclopedia
Books
· Яблоков А.В. Биология охраны природы.- Москва: Мир, 1983.-430 с.
· Новиков Г.А. Основы общей экологии и охраны природы. – Ленинград: Изд-во Ленингр. ун-та, 1979.-352 с.
· Никитин Д.П., Новиков Ю.В. Окружающая середа и человек : Учебное пособие для студентов вузов.- Москва : Высшая школа, 1986.- 415 с.
· Меренюк Г.В. Загрязнение окружающей среды и здоровье человека. – Кишинев : Штиница, 1984. – 144 с.
· Дорогунцов С.І., Коценко К.Ф., Аблова О.К. Екологія. – Київ : Либідь,1999.
· Адаменко О.М.,Косенко Я.В. Основи екології: Начальний посібник для вузів. – К.,2005.
· Білявський Г.О., Фурдуй Р.С., Костіков І.Ю. Основи екології: Підручник. – Київ : Либідь, 2005. – 408 с.
· Тучина Н.В., Меркулова Т.К., Кузьмина В.С. Speak English with pleasure. – Харьков 2003, с. 233