Рефераты по авиации и космонавтике
Рефераты по административному праву
Рефераты по безопасности жизнедеятельности
Рефераты по арбитражному процессу
Рефераты по архитектуре
Рефераты по астрономии
Рефераты по банковскому делу
Рефераты по сексологии
Рефераты по информатике программированию
Рефераты по биологии
Рефераты по экономике
Рефераты по москвоведению
Рефераты по экологии
Краткое содержание произведений
Рефераты по физкультуре и спорту
Топики по английскому языку
Рефераты по математике
Рефераты по музыке
Остальные рефераты
Рефераты по биржевому делу
Рефераты по ботанике и сельскому хозяйству
Рефераты по бухгалтерскому учету и аудиту
Рефераты по валютным отношениям
Рефераты по ветеринарии
Рефераты для военной кафедры
Рефераты по географии
Рефераты по геодезии
Рефераты по геологии
Рефераты по геополитике
Рефераты по государству и праву
Рефераты по гражданскому праву и процессу
Рефераты по кредитованию
Рефераты по естествознанию
Рефераты по истории техники
Рефераты по журналистике
Рефераты по зоологии
Рефераты по инвестициям
Рефераты по информатике
Исторические личности
Рефераты по кибернетике
Рефераты по коммуникации и связи
Рефераты по косметологии
Рефераты по криминалистике
Рефераты по криминологии
Рефераты по науке и технике
Рефераты по кулинарии
Рефераты по культурологии
Статья: Methylotrophic biomass as 2H-labeled substrate for biosynthesis of inosine
Статья: Methylotrophic biomass as 2H-labeled substrate for biosynthesis of inosine
Methylotrophic biomass as 2H-labeled substrate for biosynthesis of inosine
Oleg V. Mosin1
1 M. V. Lomonosov State Academy of Fine Chemical Technology, Vernadskogo Prospect 86, Moscow, 117571
Abstract
It was proposed to use the 2H-labeled hydrolysate of RuMP facultative methylotroph Brevibacterium methylicum, obtained from deuterated salt medium dM9 as a substrate for the growth of inosine producing bacterium Bacillus subtilis. The growth of the bacterim was performed via glucose convertion on specially developed medium dHM with 78.5% (m/m) 2H2O and supplimented with 2.5% (m/m) of 2H-labeled methylotrophic hydrolysate. To evaluate the level of deuterium enrichment FAB MS technique was used after the isolation of 2H-labeled inosine. 2H-labeled inosine obtained from dHM medium represented a mixture of molecular species containing various number of included deuterium atoms with different contribution to the enrichment. The level of enrichmet calculated by the presence of most abandant peak of the molecular ion in cluster ((M+H)+ at m/z 274) was estimated as five deuterium atoms, from which three are attributed to ribose and two to hypoxantine.
Keywords: 2H-labeled growth substrates - Bacillus subtilis - Biosynthesis - 2H-labeled inosine
Introduction
Nucleosides labeled with deuterium (2H) and other stable isotopes are becoming an indispensable tool for biomedical diagnostic and the investigation of various aspects of the metabolism [1, 2]. Thus inosine which is known as an important intermediate in the synthesis of inosine monophosphate (IMP) is in the focal point of clinical interest in medical diagnostic of heart deceases and in certain medical cases [3, 4].
There are several approaches reported for the preparation of 2H- nucleosides. Chemical synthesis are usually tedious and inefficient. Only by employing mutant forms of bacteria, which can produce a large quantities of the nucleosides when growing of an organism on media containing deuterated substrates, the desired biochemicals can be obtained both with high yields and enrichments. On the microbial production of inosine, there have been many studies so far [5-7]. .
For instance, a certain adenine, histidine and tyrosine auxotrophic mutants derived from Bacillus subtilis have been found to have a remarkable ability to produce a large amount of inosine in the growth medium, and at the present it may be produced on an industrial scale.
The major disadvantage of production of 2H-nuclesides is difficulty in obtaining the appropriate deuterated growth substrates. One approach to solve this problem is to use the extracts obtained from microorganisms growing on minimal media with 99,9 at.% 2H2O far [8]. Thus, we recently described a facultative methylotrophic bacterium Brevibacterium methylicum, which seems to be an an ideal source for the preparation of uniformelly labeled growth substrates on the basis of its 2H-biomass prepared from 2H2O and [U -2H]MetOH [9, 10]. In this article, we demonstrate the possibility of using the hydrolysates of 2H-labeled biomass of this bacterium as substrates for growing the inosine producing mutant B. subtillis.
Materials and methods
Chemicals
2H2O (99.9 at.% 2H[1]) was obtained from Russian Scientific Enterprises, Sanct Petersburg and purified by distillation from alkaline permanganate. [U -2H]methanol (95.7 at.% 2H) was from Biophysic Center, Pushino. All other chemicals were of reagent grade.
To create a high isotopic content in growth medium, 2H2O with trade marked isotopic purity 99.9 at.% 2H, was used. However, the deuterium content of used 2H2O verified by NMR was found to be 97 at.% 2H. The water containing salts were several times preliminarily crystallyzed in pure 2H2O and dried in vacuum before using (the true content of deuterium in growth media after the autoclaving was less smaller on 8-10% then isotopic purity of an initial 2H2O.
The bacterial strain
Adenine, tyrosine and hystidine auxotroph mutant B. subtilis B -3157 capable to produce and accumulate 17 g/liter of inosine during the growth on protonated medium with glucose and yeast extract was employed. The strain was obtained from Russian State Scientific Center for Genetics and Selection of Industrial Microorganisms GNIIGENETIKA.
Preparation of 2H-labeled growth substrates
The methylotrophic bacterium B. methylicum # 5662 was grown on salt medium dM9 with 93.5% (m/m) 2H2O and 2% (m/m) [U -2H]MetOH in mass culture [11]. Cells were pelleted by centrifugation (2000 g, 10 min), washed once with 2H2O and stored at -14 0C. Periodically, 10 g (wet weight) portions are thawed, suspended in 0.5 N 2HCl solution (in 2H2O) and autoclaved at 1200C for 30 min. After adjusting pH till 7.0-7.2 with potassium hydroxide, the hydrolysate was used as a mixure of 2H-labeled growth substrates for the growth of inosine producing strain.
Media and growth conditions
The bacterial growth was carried out on FM medium (m/m.%): glucose 12; yeast extract 2.5; ammonium nitrate 3; magnium sulphate 2; chalk 2. The composition of dHM was as the same as FM except dHM was prepared from 2H2O and the hydrolysate of 2H-labeled methylotrophic biomass was added. The media were sterilized by autoclaving at 1200C for 30 min and cooled. Glucose was sterilized separetely in 2H2O solution, and after that added in growth medium. рН was adjusted till 6.5-6.7 with potassium hydroxide. The bacterium was grown in 250 ml Erlenmeyer flasks containing 20 ml of the medium at 32-34 0С and vigorously aerated on an orbital shaker. After 7 days the cells were pelleted by centrifugation (2000 g, 10 min). The supernatant was separated, lyophilized and used for the isolation of 2H-labeled inosine.
Isolation of inosine
MetOH solution in H2O (50 v/v %, 20 ml) was added to a lyophilized growth medium. The mixture was allowed to - 4 0C and after 10 h the total protein was precipitated and removed by centrifugation (1200 g, 10 min). MetOH was evaporated under reduced pressure. The resulting mixture was dissolved in 2H2O (30 ml) and 5 g of activated carbon was added. After keeping for 24 h at -4 0C, the inosine, eluting with ammonia, was concentrated and twice recrystallized from MetOH (nd20 = 1.33). The purity of the product was judged by using controls of normal nucleosides, and running mixed TLC with graded amounts of the neighboring nucleosides.
Quantitative determination
During the growth inosine was separated by TLC on Silufol UV-254 plates with mobile phases: n -ButOH - AcOH - water (2:1:1, v/v) using pure commercial available inosine as a standard. The amount of inosine was determined for 10 ml aliquots of liquid growth medium by TLC. The sports were eluted by 0.1 N solution of HCl (10 ml). The absorbance of the eluates was measured at 249 nm and the content of inosine was determined using a standard curve.
The convertion of glucose was estimated enzymatically with glucoseoxydenase method [].
Equipment
Absorbance was measured with a spectrophotometer Beckman DU-6 (USA).
The analysis of protein hydrolisates was carried out using a Biotronic LC 50001 chromatograph (Germany), 230 x 3.2 mm, working pressure 50-60 atm, flow-rate 18.5 ml/h.
The levels of deuterium enrichment of amino acids were investigated with the aid of EI MS after derivatization to methyl esters of N-Dns-amino acids [].
FAB MS was performed on Hitachi MBA spectrometer (Japan) on glyserol template at potential 5 кV and an ion current of 0.6-0.8 мА.
RESULTS AND DISCUSSION
Production of 2H-labeled inosine
For biosynthesis of 2H- labeled inosine we employed bacterium Bacillus subtillis, which could produce and accumulate a conciderable amount of inosine exogeniously due to an altered nucleoside metabolism. This strain displayed the maximum productivity on FM medium, containing as a source of carbon and energy glucose (12 m/m.%), and as a source of growth factors and additional source of nitrogen the yeast extract. Since the small availability of commercial available 2H-labeled biomass prepared from yeast, it was necessary to find the more suitable microbial source, from which the 2H-labeled growth substrates could be obtained. For this purpose we employed the available RuMP facultative methylotroph Brevibacterium methylicum [5] with the content of the total protein and polycarbohydrates in biomass 53 and 10% respectively [6].
The content of amino acids in biomass of B. methylicum and the deuterium enrichment are shown in Table.
Table:
The content of amino acids in biomass of B. methylicum and the deuterium enrichment.
| Amino Acids | The content in biomass, % | Deuterium enrichment , % |
| Glycine | 9,69 | 90,0 |
| Alanine | 13,98 | 97,5 |
| Valine | 3,74 | 50,0 |
| Leucine/Isoleucine | 7,33/3,64 | 49,0 |
| Phenylalanine | 3,94 | 95,0 |
| Tyrosine | 1,82 | 92,8 |
| Serine | 4,90 | 90,0 |
| Threonine | 5,51 | not determined |
| Methionine | 2,25 | not determined |
| Aspartic Acid | 9,59 | 66,6 |
| Glytamic Acid | 10,38 | 70,0 |
| Lysine | 3,98 | 58,9 |
| Arginine | 5,27 | not determined |
| Histidine | 3,72 | not determined |
The hydrolysis of 2H-labeled biomass was performed in mild conditions via its autoclaving (30 min, 08 atm) in 0.5 N solution of 2HCl (in 2H2O). The data on the amino acid composition of hydrolysate and levels of the enrichment are shown in Fig. 2. The contents of tyrosine and histidine in hydrolysate were 1.82 and 3.72% and can ensure the polyauxotrophy of the inosine producing strain. Another important parameter is a high level of amino acid enrichment.
Bacterial growth and production of inosine
Two following media were used for the bacterial growth:
1). FM medium, prepared from ordinary protonated water and yeast extract.
2). dHM medium, prepared from 87.5% (v/v) 2H2O and 2.5% (m/m) of 2H-labeled methylotrophic hydrolisate, obtained accordingly from medium dМ9.
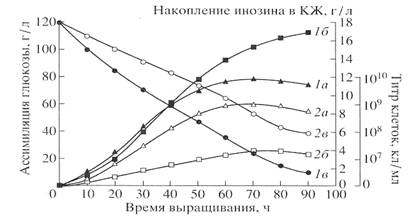
Fig.1
Curves, reflecting the growth dynamics (a), convercion of glucose (b) and production of inosine (c) are given in Fig. 1. A maximal level of inosine production on ordinary protonated medium was 17 g\liter. When growing on dHM medium the strain produced only 3.9 g/liter of inosine throughout the whole course of the growth. The low level of inosine production was correlated with a degree of glucose conversion in those conditions. 4m/m.% of non-assimilated glucose was detected in medium dHM after the growth, that proved that glucose is metabolized less effectivelly on medium dHM, that is probably a result of non-equvalent replacement of yeast extract by methylotrophic hydrolysate.
The absorption spectra of inosine isolated from medium dHM (a) are shown in Fig. 2 comparatively to the growth medium (b) and commertially available inosine (c). TLC of isolated inosine showed the presence of main spot with Rf=0.5 (inosine) and additional spot with Rf=0.75 (hypoxantine). The output of 2H-labeled inosine was 1 gram from 1 liter of growth medium.
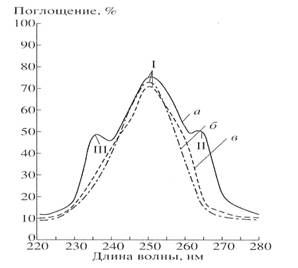
Fig.2
The evaluation of inosine enrichment
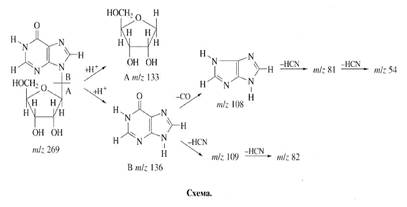
The method of FAB MS was employed for the evaluation of inosine enrichment. The fragmentation pathways of inosine by FAB MS are shown in Fig. 3. Two main decomposition processes arised from the molecule: sugar (m/z 133) and hypoxantine (m/z 136) formation. The compounds with a smaller m/z ratio may further to be formed as a result of elitination of HCN and CO from hypoxantine. The level of deuterium enrichment could be evaluated from the FAB mass spectrum of 2H-labeled inosine shown in Fig. 3, b compared with the non-labeled inosine (a). The results, firmely established the labeling of inosine as heterogenious, juging by the presence of clasters of adduct peaks at the molecular ion MH+; the species of molecules with different numbers of deuterium atoms were visualised. The most abundant peak with (M+H)+ at m/z 274 (instead of m/z 269 for non-labeled compound) in the claster was registered by mass spectrometer as a peak with average m/z ratio, from whom the enrichment of inosine was calculated as five deuterium atoms. The presence of peak corresponding to the hypoxantine fragment [C5H4ON4]+ at m/z 138 (instead of m/z 136) and the peak of sugar fragment [C5H9O4]+ at m /z 136 (instead of m/z 133) proved that two deuterium atoms are located in hypoxantine, however, three of them are attributed to the ribose pattern.
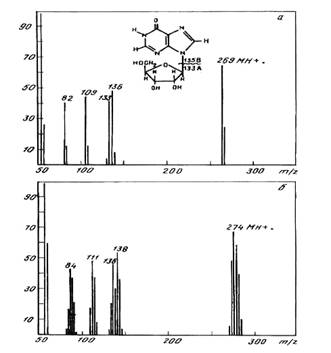
Fig.3
Mainly two aspects of the enrichment of inosine were taken into account (scheme). First, because protons in С’1-С’5 positions of ribose pattern in inosine could be originated from glucose, we assumed, that the character of biosynthetic enrichment of deuterium in sugar pattern of inosine is determined mainly to the functioning of a number of processes of hexose monophosphate shunt of glucose assimilation. But since protonated glucose was added in growth medium, its contribution in the inosine enrichment was minimal. Nevertheless, the results suggested, that ribose contained three deuterium atoms that could not stemp from glucose. Three deuterium atoms probably stemp via some minor reactions of glucose biosynthesis. Secondly, the numerous exchange processes and intermolecular regrouping reactions, occurring with participation of 2H2O could also be resulted in specific labelling of inosine. Such accessible positions are occupied by the easily exchangeable hydrogen (deuterium) atoms both of hydroxylic- and imino groups of inosine. Two protons at C-H positions in inosine could be replaced by deuterium via assimilation of 2H-labeled hydrolysate. The enrichment of inosine was approximately the same as 2H2O content in growth medium (65.5-67.5%).
LITERATURE.
1. Munch-Petersen A., (1983) Metabolism of nucleotides, nucleosides, and nucleobases in microorganisms. Academic Press. Inc., New York. 105.
2. Wuthrich K. (1986) NMR of proteins and nucleic acids. New York: J. Wiley & Sons. 14.
3. Bloch A. (1975) Chemistry, biology, and clinical uses of nucleoside analogs. Academic Press, New York. 58.
4. Farber E., Shull H., McConomy J.M., and Castillo A.E. (1965) Biochem. Pharmacol. 14, 761.
5. V.I. Shvets, A.M. Yurkevich, O.V. Mosin, D.A. Skladnev. (1995) Karadeniz Journal of Medical Sciences. 8. No 4. P.231-232.
6. Ishii K., & Shiio I., (1972) Agric. Biol. Chem. 36, 1511-1522.
7. Matsui H., Sato K., Enei H., and Takinamy K., (1982) Agric. Biol. Chem. 46, 2347-2352.
8. Katz J. & Crespi H.L. (1972) Pure Appl. Chem. 32, 221-250.
9. Mosin O.V., Karnaukhova E.N., Pshenichnikova A.B., et al. (1993) Biotechnology (Russia). 9, 16-20.
10. Egorova T.A., Mosin O.V., Eremin S.V., Karnaukhova E.N., Zvonkova E. N., Shvets V.I. 91993) Biotechnology (Russia) 8, 21-25.